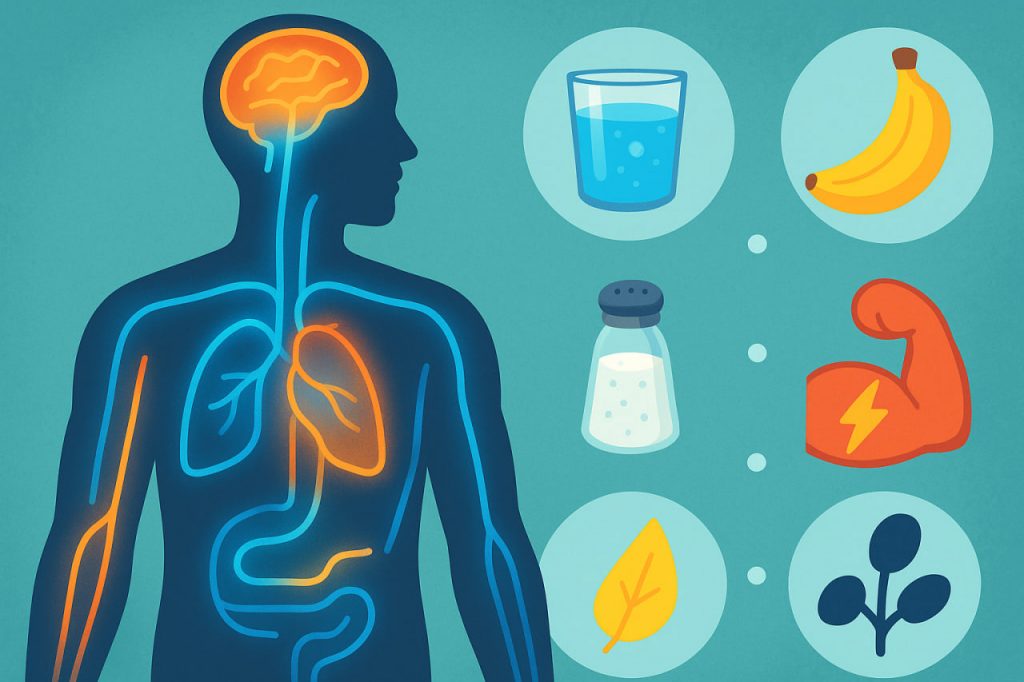
Electrolytes are vital substances that keep our bodies functioning correctly. They are minerals that carry an electric charge when dissolved in water. These charged particles play a critical role in maintaining hydration, nerve function, muscle contractions, and pH balance. Without proper electrolyte levels, even simple bodily processes — like moving, thinking, or staying awake — would not be possible.
What Are Electrolytes?
Electrolytes are minerals such as sodium, potassium, calcium, magnesium, chloride, phosphate, and bicarbonate. They are present in blood, tissues, and body fluids. When these minerals dissolve in water, they form positive and negative ions that help transmit electrical impulses throughout the body.
For example:
- Sodium (Na⁺) regulates water balance and nerve signaling.
- Potassium (K⁺) supports heart rhythm and muscle function.
- Calcium (Ca²⁺) strengthens bones and helps muscles contract.
- Magnesium (Mg²⁺) aids enzyme function and reduces muscle cramps.
- Chloride (Cl⁻) maintains acid-base balance and fluid stability.
Why Electrolytes Are Important
- Hydration and Fluid Balance
Electrolytes control how much water enters and leaves cells. Without them, the body cannot retain fluids properly, leading to dehydration or swelling. - Nerve Transmission
Every nerve signal in the human body depends on an electrical charge — and electrolytes provide that charge. They allow communication between the brain, muscles, and organs. - Muscle Function
Proper levels of potassium, calcium, and magnesium ensure smooth muscle movement, including the beating of the heart. An imbalance can cause cramps, weakness, or irregular heart rhythms. - pH Regulation
The body must maintain a slightly alkaline pH (around 7.4). Electrolytes, especially bicarbonate and phosphate, act as buffers that prevent harmful acidity or alkalinity. - Energy and Recovery
Electrolytes are essential for athletes and active individuals. They help replace minerals lost through sweat, preventing fatigue and maintaining endurance during physical activity.
What Causes Electrolyte Imbalance
An imbalance can occur due to:
- Dehydration from excessive sweating, diarrhea, or vomiting.
- Overhydration (drinking too much water without minerals).
- Kidney disease, which disrupts fluid regulation.
- Certain medications, such as diuretics or laxatives.
Symptoms of imbalance include:
- Dizziness or headaches
- Muscle cramps or weakness
- Rapid heartbeat
- Confusion or irritability
- Fatigue or nausea
Severe cases may require medical intervention, especially if caused by kidney or metabolic disorders.
How to Maintain Healthy Electrolyte Levels
- Drink balanced fluids — water with added minerals or electrolyte drinks.
- Eat mineral-rich foods like bananas (potassium), dairy (calcium), nuts (magnesium), and sea salt (sodium).
- Avoid excessive processed foods, which may contain too much sodium but lack other vital minerals.
- Rehydrate after exercise or illness with electrolyte solutions or homemade mixes (water, lemon juice, salt, honey).
Electrolytes Beyond Biology
Electrolytes are also used in science and technology — for example, in batteries, electrolysis, and medical equipment that monitors heart and muscle activity. They act as energy carriers both inside living organisms and in engineered systems.
Interesting Facts
- The human brain uses electrical impulses powered by electrolytes to send signals through neurons.
- Coconut water is often called “nature’s sports drink” because it naturally contains potassium, magnesium, and sodium.
- Severe electrolyte imbalance can cause cardiac arrest due to disrupted electrical signals in the heart.
- The word electrolyte comes from Greek roots meaning “loosened by electricity.”
- Modern sports science uses isotonic drinks that mimic the body’s natural electrolyte concentration.
Glossary
- Ion — an atom or molecule with an electric charge.
- Isotonic drink — a fluid with the same salt and sugar concentration as the human body.
- Electrolysis — a process that uses electric current to cause a chemical change.
- Homeostasis — the body’s ability to maintain stable internal conditions.
- Buffer — a substance that resists changes in pH levels.